
4/1/2004
Tacrolimus Ointment Demonstrates Superior Efficacy over Pimecrolimus Cream in the Treatment of Atopic Dermatitis
Expert Commentary
Alan B. Fleischer, Jr., MD, Professor and Chair of Dermatology, Wake Forest University School of Medicine, Winston-Salem, North Carolina
According to the results of 3 studies presented during the 2004 American Academy of Dermatology 62nd Annual Meeting, tacrolimus ointment is more effective than pimecrolimus cream in the treatment of atopic dermatitis in both pediatric and adult patients.1 Moreover, tacrolimus and pimecrolimus demonstrated similar safety profiles with no statistical difference in cutaneous or systemic adverse events. The 3 studies represent the first, adequately powered, direct comparative trials examining tacrolimus and pimecrolimus for the treatment of atopic dermatitis in the same patient population including age, disease severity, duration of treatment, efficacy endpoints and adverse event coding scheme.
Atopic dermatitis is a chronic relapsing inflammatory skin disease characterized by xerosis, pruritus, inflammation, and exudation.2,3 The incidence of atopic dermatitis has increased dramatically over the last several decades.4,5 Atopic dermatitis has been reported to affect more than 10% of children and adolescents,6 with approximately 60 to 70% of patients experiencing symptoms into adulthood.7 The precise etiology of atopic dermatitis has not been fully elucidated, but accumulating evidence suggests a multifactorial cause, with activation of complex immunologic and inflammatory pathways.8
Although there is no cure for atopic dermatitis, available treatment options focus on symptom relief.4 Topical corticosteroids have been the mainstay of treatment for atopic dermatitis and are used intermittently to reduce inflammatory flares. However, prolonged treatment with topical corticosteroids has been associated with significant side effects such as skin atrophy and suppression of the hypothalamic-pituitary-adrenal axis.4,8
Tacrolimus was the first topical immunomodulator and is approved for the treatment of moderate to severe atopic dermatitis in patients aged 2 years and older.9 Tacrolimus targets the phosphatase activity of calcineurin, ultimately blocking expression of cytokines central to initiating an immune response.10 Multiple clinical trials with tacrolimus, involving more than 13,000 children and adults, have shown that tacrolimus is well tolerated and highly effective at improving atopic dermatitis.10 Importantly, tacrolimus administration is not associated with skin atrophy, adrenal suppression, or systemic immunosuppression, as has been observed with topical corticosteroids.5,10
Pimecrolimus is another topical immunomodulator and is approved for the treatment of mild to moderate atopic dermatitis in patients aged 2 years and older.11 Pimecrolimus has the same mechanism of action as tacrolimus, and its efficacy and safety in treating atopic dermatitis has been shown in several clinical trials.12,13
Until now, there have been few reports of direct head-to-head trials comparing tacrolimus and pimecrolimus in the treatment of atopic dermatitis. Comparisons between these two agents have relied on interpreting the published results of clinical trials that were designed to examine each agent against placebo or topical corticosteroids. Because these trial designs varied in patient population, duration of treatment, length of follow-up, and endpoints evaluated, these comparisons are limited and cannot adequately assess potential differences between tacrolimus and pimecrolimus therapy.
This Dermatology Express Report™ will review the results of 3 direct comparative clinical trials that were specifically designed to standardize treatment parameters in evaluating the efficacy and safety of tacrolimus and pimecrolimus in the treatment of atopic dermatitis.
Tacrolimus Ointment Demonstrates Superior Efficacy over Pimecrolimus Cream in Atopic Dermatitis
A total of 837 patients with atopic dermatitis were evaluated in three 6-week, randomized, investigator-blinded, studies evaluating tacrolimus ointment and pimecrolimus cream. Patients were stratified in 3 populations: adult (≥16 years, n = 350) patients with mild to very severe atopic dermatitis randomized to tacrolimus ointment 0.1% or pimecrolimus cream 1%; pediatric (≥2 years to <16 years, n = 198) patients with moderate to very severe atopic dermatitis randomized to tacrolimus ointment 0.1% or pimecrolimus cream 1%; and pediatric (≥2 years to <16 years, n = 289) patients with mild atopic dermatitis randomized to tacrolimus ointment 0.03% or pimecrolimus cream 1%.
All patients were evaluated on the same efficacy and safety endpoints. Endpoints included the percent change from baseline in the Eczema Area and Severity Index (EASI) and the physician-rated Body Surface Area (BSA) assessment, patient assessment of itch, and the six-point Investigator's Global Atopic Dermatitis Assessment (IGADA) (endpoint = 0, "clear" or 1, "almost clear"). Assessments for efficacy and safety were conducted on Day 1, Week 1, Week 3, and Week 6 (end of study). Adverse events, using the same coding system for all patients, were recorded throughout the treatment period of 6 weeks.
Each study design consisted of twice daily application of therapy for 6 consecutive weeks. However, if skin lesions cleared, treatment was continued for one additional week after which end-of-study evaluations were performed. Concomitant topical medications were not allowed during the study. However, patients were allowed to use non-medicated emollients on non-treatment areas, as well as sunscreen.
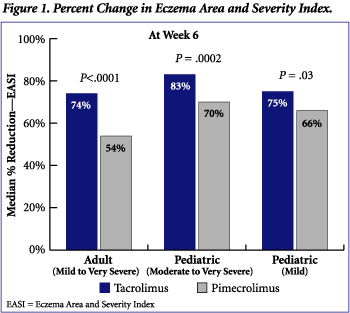
Analysis of all efficacy endpoints indicated that treatment with tacrolimus ointment was superior to pimecrolimus cream. The median percent reduction in the EASI at Week 6 (end of study) was greater for tacrolimus than pimecrolimus in all treatment groups (Figure 1). Adult patients treated with tacrolimus experienced a median reduction in EASI of 74% compared to 54% in adult patients treated with pimecrolimus (P<.0001). Similarly, median reductions in EASI from baseline for pediatric patients (moderate to very severe atopic dermatitis) treated with tacrolimus were 83% compared to 70% in patients treated with pimecrolimus (P = .0002). A significant difference in EASI reduction was also observed in pediatric patients (mild atopic dermatitis) who received tacrolimus (75% reduction) relative to those who received pimecrolimus (66% reduction) (P = .03).
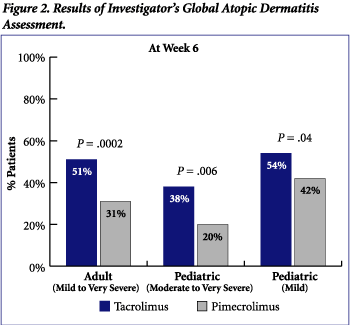
At Week 6 (end of study), the percent of patients with "clear" or "almost clear" disease, as determined by the IGADA, were statistically significantly higher for all patients treated with tacrolimus compared to pimecrolimus (Figure 2). Fifty-one percent of adult patients treated with tacrolimus had "clear" or "almost clear" skin compared to 31% of pimecrolimus-treated patients (P = .0002). A greater percentage of pediatric patients (moderate to very severe atopic dermatitis) treated with tacrolimus had "clear" or "almost clear" skin compared to pimecrolimus-treated patients (38% vs 20%; P = .006). For pediatric patients with mild atopic dermatitis, treatment with tacrolimus resulted in 54% of patients with "clear" or "almost clear" skin compared to 42% with pimecrolimus treatment (P = .04).
Adult and pediatric patients with mild/moderate to very severe atopic dermatitis treated with tacrolimus achieved statistically significantly higher percent reductions from baseline in BSA compared to patients treated with pimecrolimus. Tacrolimus improved BSA by 68% in both adult and pediatric patients with mild/moderate to very severe atopic dermatitis, whereas pimecrolimus improved BSA in 48% and 41% of adult and pediatric patients with mild/moderate to very severe atopic dermatitis, respectively (P = .0002).
Tacrolimus was statistically significantly superior to pimecrolimus in improving itch scores in both adult and pediatric patients with mild/moderate to very severe atopic dermatitis (Figure 3). The median percent reduction in itch scores for adults treated with tacrolimus was 69% compared to 52% with pimecrolimus (P = .04). Pediatric patients with moderate to very severe atopic dermatitis also experienced improved itch scores (67%) with tacrolimus that were superior to improvements observed with pimecrolimus (43%) (P = .005).
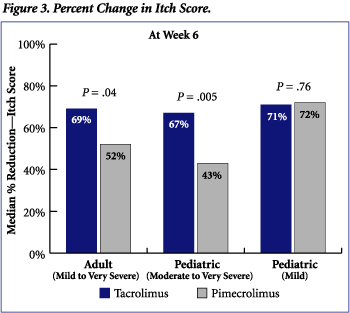
Differences in BSA and itch scores were not statistically significant between tacrolimus and pimecrolimus therapy in pediatric patients with mild atopic dermatitis.
Adverse Events
There were no statistical differences between tacrolimus ointment and pimecrolimus cream with respect to any adverse event. The most frequent treatment-emergent adverse events observed were local burning and stinging, followed by increased itch (Figure 4).

Withdrawal from Treatment
Patient withdrawals due to lack of therapeutic effect or adverse events were higher in all patients treated with pimecrolimus. Withdrawals due to lack of efficacy were 2-fold higher among pediatric patients with moderate to very severe atopic dermatitis treated with pimecrolimus compared to tacrolimus (8% vs 4%, respectively), and 8 times higher for pediatric patients with mild atopic dermatitis treated with pimecrolimus compared to tacrolimus treatment (8% vs 1%, respectively). In addition, withdrawals due to adverse events were consistently higher in pediatric patients treated with pimecrolimus: 7% of patients with moderate to very severe atopic dermatitis and 6% of patients with mild atopic dermatitis discontinued due to adverse events compared to 4% and 1% of tacrolimus-treated patients, respectively. Two percent of adult patients with mild to very severe atopic dermatitis withdrew due to lack of tacrolimus efficacy compared to 5% with pimecrolimus, while 3% of tacrolimus-treated adult patients and 4% of pimecrolimus-treated adult patients discontinued prematurely due to adverse events.
Conclusion
In 3 randomized, head-to-head comparative studies, tacrolimus ointment was shown to be superior to pimecrolimus cream for the treatment of atopic dermatitis in adult and pediatric patients. Moreover, tacrolimus and pimecrolimus demonstrated similar safety profiles with no statistical difference in cutaneous or systemic adverse events.
References
1. Tacrolimus ointment (Protopic®) vs. pimecrolimus cream (Elidel®) for treatment of atopic dermatitis. Presented in Symposium 302 "New and Emerging Therapies", February 6, 2004 and Symposium 336 "Atopic Eczema & Other Dermatitides", February 10, 2004 at the American Academy of Dermatology 62nd Annual Meeting, February 6-11, 2004, Washington, DC.
2. Ellis C, Luger T. International Consensus Conference on Atopic Dermatitis II (ICCAD II): Chairman's introduction and overview. Br J Dermatol. 2003;148 (Suppl 63):1-2.
3. Ahuja A, Land K, Barnes CJ. Atopic dermatitis. South Med J. 2003;96:1068-1072.
4. Ellis C, Luger T, Abeck D, et al. International Consensus Conference on Atopic Dermatitis II (ICCAD II): clinical update and current treatment strategies. Br J Dermatol. 2003;148(Suppl 63):3-10.
5. Stone KD. Atopic diseases of childhood. Curr Opin Pediatr. 2003;15:495-511.
6. Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103:125-138.
7. Lammintausta K, Kalimo K, Raitala R, Forsten Y. Prognosis of atopic dermatitis. A prospective study in early adulthood. Int J Dermatol. 1991;30:563-568.
8. Novak N, Bieber T, Leung DY. Immune mechanisms leading to atopic dermatitis. J Allergy Clin Immunol. 2003;112(6 Suppl):S128-S139.
9. Protopic prescribing information [package insert]. Fujisawa Healthcare, Inc. Accessed March 12, 2004.
10. Nghiem P, Pearson G, Langley RG. Tacrolimus and pimecrolimus: from clever prokaryotes to inhibiting calcineurin and treating atopic dermatitis. J Am Acad Dermatol. 2002;46:228-241.
11. Elidel prescribing information [package insert]. Novartis Pharmaceuticals Corp. Accessed March 12, 2004.
12. Eichenfield LF, Lucky AW, Boguniewicz M, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol. 2002;46:495-504.
13. Wahn U, Bos JD, Goodfield M, et al for the Flare Reduction in Eczema with Elidel (Children) Multicenter Investigator Study Group. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110(1 Pt 1):e2.
Disclosure
Alan B. Fleischer, Jr., MD
Grant/Research Support-Fujisawa Healthcare, Inc., Novartis;
Speakers Bureau-Fujisawa Healthcare, Inc.
This report contains no information on commercial products that are unlabeled for use or investigational uses of products not yet approved.
Dermatology Express Report™ is a product of Millennium Medical Communications, Inc. ("MMC, Inc."), an independent, third-party organization providing educational information concerning current medical data and opinions presented at worldwide medical meetings. The Dermatology Express Report™ is published in accordance with the Guidance for Industry: Industry Supported Scientific and Educational Activities, 62 Fed. Reg. 64,093, 64,096-99 (1997) adopted by the U.S. Department of Health and Human Services Food and Drug Administration. Pursuant to the foregoing standards, MMC, Inc. is solely responsible for selecting the topics discussed herein. This Dermatology Express Report™ may contain data on products, product uses, indications, and dosages which are not approved for use in the USA, Canada, and the European Union and no endorsement is hereby made or intended by coverage of any unapproved use. The content of this report is intended for educational purposes only, and merely conveys scientific data presented at medical meetings. Approved product labeling should always be consulted for prescribing information. The Dermatology Express Report™ is an independent and non-promotional report intended to provide accurate scientific and medical information for educational purposes. MMC, Inc. is not responsible for errors or omissions in reports. The production of this report was supported by a wholly unrestricted educational grant from Fujisawa Healthcare, Inc. Fujisawa Healthcare, Inc. maintains no control, direct or indirect, over the content, substance, or distribution of this report.
Categories
- Allergy
- Alcohol Addiction
- Anxiety
- Cardiology
- Depression
- Dermatology
- Endocrinology
- Phlebology
- Diabetes
- Herpes Viruses
- Gastroenterology
- General Health
- Gerontology
- Hematology
- Hepatology
- Immunology
- Infectious Diseases
- Men's Health
- Neurology
- Obesity
- Oncology
- Ophthalmology
- Orthopedics & Sports Medicine
- Parasitic Diseases
- Pediatrics
- Psychiatry
- Radiology
- Respiratory
- Rheumatology
- Smoking Cessation
- Urology
- Women's Health